Abstract
Introduction: Thrombotic microangiopathy (TMA) is a clinical syndrome that can develop after hematopoietic cell transplantation (HCT). Previous studies have been limited to small case series with conflicting results. In this large, single-center longitudinal TA-TMA database, we describe the incidence, etiology, treatment, and outcome of this rare but challenging clinical presentation.
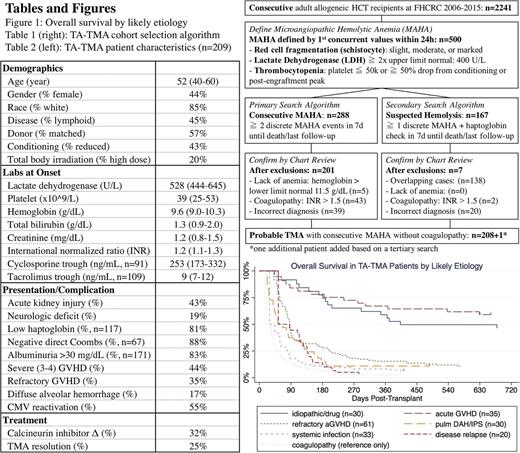
Methods: We performed a retrospective cohort analysis of consecutive allogeneic HCT recipient during 2006-2015 at Fred Hutchinson Cancer Research Center. TA-TMA was defined as persistent microangiopathic hemolytic anemia (MAHA) composed of red cell fragmentation, elevated lactate dehydrogenase (LDH), thrombocytopenia, anemia, and lack of coagulopathy (Table 1). We performed a primary search algorithm using "consecutive MAHA" followed by a secondary search algorithm using "suspected hemolysis" to identify all cases from date of transplant until date of death or last follow-up (Table 1). For each suspected case, we further reviewed available patient records to confirm the diagnosis, determine the etiology, treatment, and complication. The most likely etiology was determined by reviewing the clinical events in the 2-week window before the TMA onset. Since 96% of patients were receiving calcineurin inhibitor (CNI), we chose idiopathic/drug as a diagnosis of exclusion if all other causes were excluded. TMA resolution was defined as LDH <1.5x ULN, platelet transfusion independence, and rare or no schistocytes. Continuous and categorical variables were shown as median (interquartile range) and percentage, respectively. Overall survival was depicted with Kaplan-Meier curves, after accounting for delayed entry with left truncation at TMA diagnosis. Cox regression model was built to test the association between TMA resolution as a time-varying covariate and overall survival.
Results: Among 2241 consecutive adult allogeneic HCT recipients, 209 patients (9%) met the laboratory criteria of TA-TMA (Table 2) with a median onset time of 62d (35d-93d). One hundred and nine patients (52%) had end-organ damage with either acute kidney injury (AKI) or neurologic deficit. The median follow-up was 181d (92d-478d) and 53 patients (25%) achieved hematologic resolution. Diagnostically, acute GVHD (n=96) represented the largest category and those with GVHD refractory to high dose steroid (n=61) had poor prognosis. Diffuse alveolar hemorrhage (DAH) and idiopathic pneumonia syndrome (IPS) (n=30) also frequently preceded the TMA onset. Finally, despite excluding patients with coagulopathy, non-specific causes of TMA such as disease recurrence (n=20) and systemic infection (n=33) were frequently evident.
Overall survival, separated by TMA etiology is shown in Figure 1. Whereas the idiopathic/drug and non-refractory acute GVHD categories approached the survival of non-TMA post-HCT recipients, the other groups had worse prognosis. Regardless of cause, the most common treatment (32%) was CNI cessation or CNI switch (in no particular pattern). We did not observe an association between changes to CNI therapy and TMA resolution (24% vs. 26%, Fisher P 0.80) or overall survival at 6 months (37% vs. 34%, Wald P 0.75). This remained true when each diagnostic category and each CNI drug was separately analyzed. Plasma exchange was rarely performed at our center. Finally, TMA resolution often correlated with improvement of the underlying disorder. The achievement of TMA resolution was associated with significantly improved survival (HR 0.35, P <0.01).
Conclusion: Despite meeting the same laboratory definition for persistent MAHA, clinical review revealed heterogeneous disease groups with dissimilar prognostic implications. Our data suggest that TA-TMA is not one disease entity but rather a "final common pathway" for several disorders that likely have different pathophysiologic mechanisms. Instead of empiric treatment with CNI adjustment, we need to identify and understand the underlying causal pathway towards TA-TMA, and to determine if earlier recognition and successful treatment improves patient outcomes.
Lee: Kadmon: Other: One-time advisory board member; Amgen: Other: One-time advisory board member; Bristol-Myers-Squibb: Other: One-time advisory board member; Mallinckrodt: Honoraria. Gopal: Seattle Genetics: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal